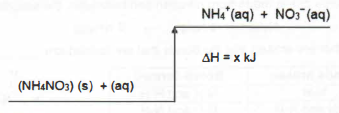
1. Carbon monoxide is used to make phosgene, COCl2, which is an important reactant in industries to make polymers. dyes and pharmaceuticals.
Phosgene was first made in 1812 by using a photochemical reaction in which a mixture of carbon monoxide and chlorine was exposed to bright sunlight. This reaction is exothermic and equation is given below.
CO + Cl2 ⇌ COCl2
(a) (i) Explain what a photochemical reaction is.
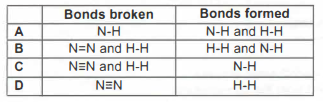
(ii) Explain, in terms of bond breaking and bond making, why the formation of phosgene is an exothermic reaction.
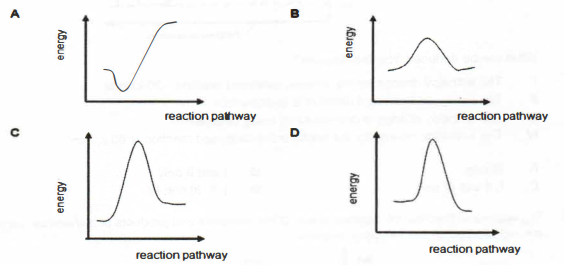
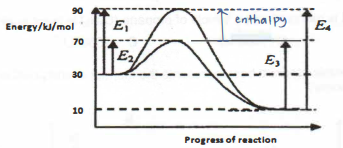
(iii) Draw an energy profile diagram for the decomposition of phosgene. On your diagram, label the activation energy for the reaction.
2. A student investigated the reaction between aqueous copper(II) sulfate and two different metals P and Q. A 25.0 cm3 sample of aqueous copper(II) sulfate was added to a Styrofoam cup from a measuring cylinder. The temperature of the solution was taken every 30 seconds. At 1.5 minutes, excess of powdered metal P was added to the cup and the mixture was stirred with a thermometer. The temperature of the solution was taken every 30 seconds. The experiment was repeated using excess of powdered metal Q. The results of the two experiments were plotted on a graph.
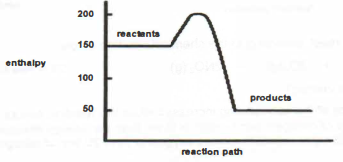
(a) is the reaction between aqueous copper(II) sulfate and metal P exothermic or
endothermic? Support your answer with evidence from the graph.
(b) Sketch a labelled energy profile diagram for the reaction between aqueous copper(II) sulfate and metal P.
(c) Deduce from the information provided which reaction would have a larger enthalpy change. the reaction between aqueous copper(II) sulfate and metal P and metal Q. Explain your answer.
(d) Describe two changes you would observe in both reactions.
(e) At the end of the experiments, it was found that 0.6609 of pure copper residue was formed. Calculate the initial concentration, in mol/dm3. of aqueous copper(II) sulfate.
Answer:



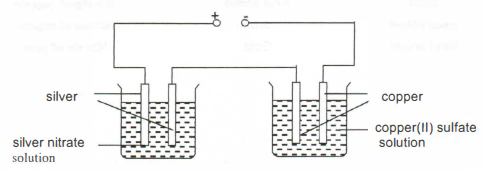
 A summary of the voltmeter readings obtained is shown in the table below.
A summary of the voltmeter readings obtained is shown in the table below. (a) Give a reason why the metal strips and copper plate must first be cleaned
(a) Give a reason why the metal strips and copper plate must first be cleaned







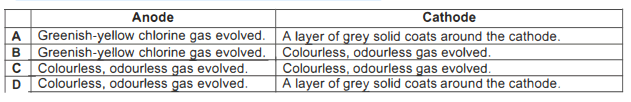
 Which of the following statements is incorrect?
Which of the following statements is incorrect?
 Which statement about the reactions is true?
Which statement about the reactions is true?