1. Which statements are true about the diagram below?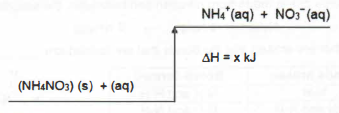
l the value of AH is negative
ll dissolving ammonium nitrate is an endothermic process
Ill during the reaction, heat is absorbed from the surroundings
A land ll only
B II and III only
C land lll only
D I, II and III
2. The equations for three reactions are given below.
Reaction 1 H2 –> 2H
Reaction 2 HCl –> H + Cl
Reaction 3 2H + O –> H2O
Which reaction/s is/are exothermic?
A Reaction 3 only.
B Reaction 1 and 2 only.
C Reaction 1 and 3 only.
D Reaction 2 and 3 only.
3. When ammonia gas is made from nitrogen and hydrogen, the equation for the reaction is: N2(g) + 3 H2(g) —> 2 NH3(g)
The bonds that are broken and the bonds that are formed are ____________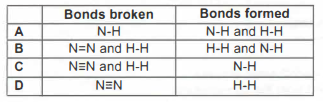
4. Hydrogen reacts with chlorine as follows.![]() The H-H bond energy is 436 kJ/mol and the Cl-Cl bond energy is 242 kJ/mol.
The H-H bond energy is 436 kJ/mol and the Cl-Cl bond energy is 242 kJ/mol.
What is the H-Cl bond energy?
A 247 kJ
B 431 kJ
C 494 kJ
D 862 kJ
5. Which energy profile diagram best represents the thermal decomposition of
copper(II) carbonate?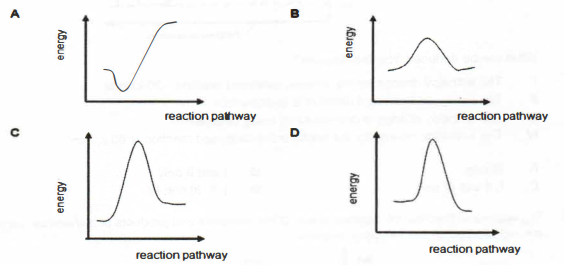
6. The diagram shows the energy profile of a reversible reaction that occurs with and without a catalyst.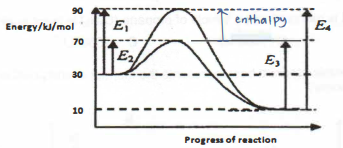
What can be deduced from the diagram?
I The enthalpy change for the fon/vard catalysed reaction —20 kJ/mol.
II The catalysed backward reaction is endothermic.
III The enthalpy change is decreased by using catalyst.
IV The activation energy for the backward uncatalysed reaction is 80 kJ/mol
A III only
B l and ll only
C I, II and IV only
D I, II, III and IV
7. The relative enthalpies, on arbitrary scale, of the reactants and products of a chemical reaction, are represented on the following diagram.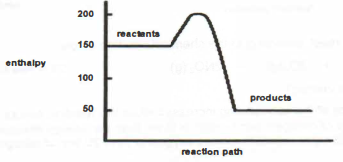
Which statements are true for the above energy profile diagram of this reaction?
I The reaction is endothermic.
II The energy absorbed to break the bonds is greater than energy released during bond formation.
III The numerical value of the activation energy of the reaction is 50.
IV The numerical value of enthalpy change of the reverse reaction is 100.
A III and IV
B II, III and IV
C I, II and Ill
D I, II, III and IV
Answer:
1. B
2. A
3. C
4. B
5. C
6. C
7. A