Learning Objectives:
- Define and classify a molecule and an atom.
- Use and interpret atomic symbols, e.g. for C.
- Define proton/atomic numbers and nucleon/mass numbers.
- State the relative charges, and approximate relative masses of
protons, neutrons and electrons. - Deduce the numbers of protons, neutrons and electrons given
the proton/atomic and nucleon/mass number.
Atoms are the smallest particle of an element that retain the chemical properties of the element. The idea of an atom is first proposed by John Dalton in the early 1800s. Dalton proposed that each chemical element is composed of a single and unique type of atom, which cannot be altered or destroyed by chemical means and can be combined to form more complex structure.

Atoms of the various elements can be joined together, through chemical means, to form a molecule. Hence, a molecule is a group of two or more atoms that are chemically combined together.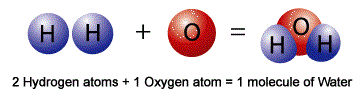
Today, we know that an atom is made up of 3 distinct sub-atomic particles: proton, neutron and electron.
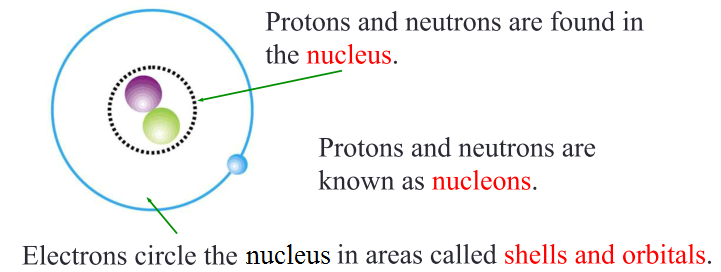
The nucleus is tiny compared to the overall size of an atom.
Protons, neutrons and electrons are very light in weight and have different physical properties from each other. Hence, scientists measure masses of atom against a standard unit called atomic unit mass (amu) whereby 1 amu = 1.67 x 10-27 kg (also known as the relative mass).

The number of protons in an atom is called the proton number or atomic number of the atom and can be represented by Z (capital z).
The total number of protons and neutrons in the nucleus of an atom is called the nucleon number (represented by A) or nucleon number. This also tells you the relative mass of an atom relative to another.
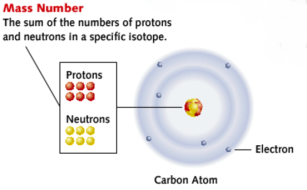
As all atoms are electrically neutral, an atom contains an equal number of positively charged proton and negatively charged electron. This also means that you can tell the number of electrons by looking at the proton (or atomic) number.
Each element is made up of different number of sub-atomic particles along with a unique atomic symbol. Hence, no 2 elements have the same proton number. All this information can be found in the periodic table.
The periodic table is a list of elements arranged in the order of increasing proton (atomic) mass.
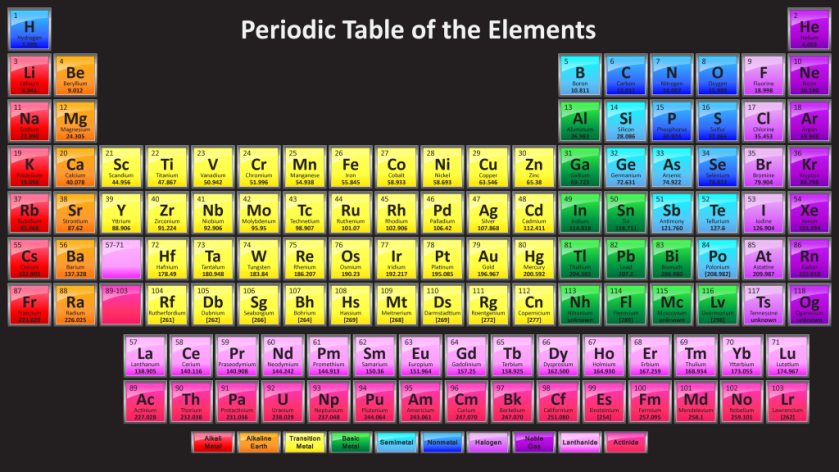
How do we read the periodic table?

The elements are arranged into periods and groups. The periodic table has 8 groups of elements which is numbered from I, II, … to 0 and each element in a period has a proton number which is one less than the element after it.
Activity:
1. The information below is from part of the Periodic Table of Elements.Use this information to answer the questions that follow.
2. An atom of iodine (I) has an atomic mass of 127 amu. How many protons and neutrons does a iodine atom contain?
________ protons
________ neutrons
3. What is the total atomic mass of the water molecule?
____________